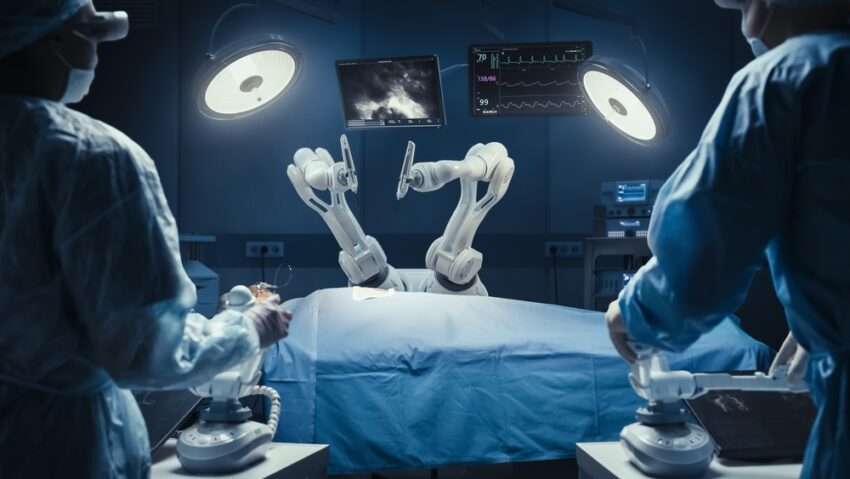
CMR Surgical, based in Cambridge, has received FDA approval for its Versius robotic system, a move set to open new opportunities in the US market.
- The Versius system, noted for mimicking human arm movements, aims to enhance surgical precision and is the second most widely used globally.
- Founded in 2014, CMR has raised significant funds and employs over 500 staff, primarily in the UK.
- This FDA approval marks a pivotal step for the company’s international expansion, targeting major markets like Japan and China.
- CMR’s strategic growth includes potential future IPO plans, supported by investors like SoftBank and Tencent.
CMR Surgical, a Cambridge-based firm, has achieved a significant milestone by securing approval from the US Food and Drug Administration (FDA) for its Versius robotic surgical system. This development is expected to facilitate the company’s entry into the US market, initially focusing on gallbladder removal surgeries for adult patients aged 22 and above. The Versius system, designed to replicate human arm movements, enhances the precision of procedures and is already the second most popular surgical robot globally, with over 26,000 surgeries completed, including numerous operations in the UK.
Founded nearly a decade ago in 2014, CMR Surgical has demonstrated remarkable growth and innovation in the field of medical robotics. The company is headquartered in Cambridge and receives backing from prominent international investors, such as the Japanese technology firm SoftBank and China’s Tencent. Since its inception, CMR has raised approximately $1 billion in funding, including a landmark $600 million round in 2021 led by SoftBank, marking the largest private investment in the medtech sector globally. This robust financial support has enabled CMR to employ over 500 staff, most of whom are stationed in the UK.
Mark Slack, CMR’s chief medical officer and co-founder, highlighted the significance of this FDA approval, stating, “Securing FDA marketing authorisation for Versius is a significant milestone for CMR and, most importantly, for hospitals and patients who will now have greater access to robotic-assisted surgery.” Beyond the United States, CMR is actively seeking regulatory approvals in other major healthcare markets, including Japan and China, to further its mission of advancing robotic-assisted surgery worldwide.
While CMR Surgical has not formalised plans for an initial public offering (IPO), it remains a viable option as the company continues its expansion into key international markets. The strategic growth of CMR, coupled with its innovative robotic system, positions the company as a formidable competitor in the growing market for medical robotics. The recent FDA approval is poised to significantly bolster CMR’s presence and operations on the global stage.
The FDA’s approval of the Versius system significantly enhances CMR Surgical’s prospects in the US and global markets.